Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans
Abstract
:1. Introduction
2. Results and Discussion
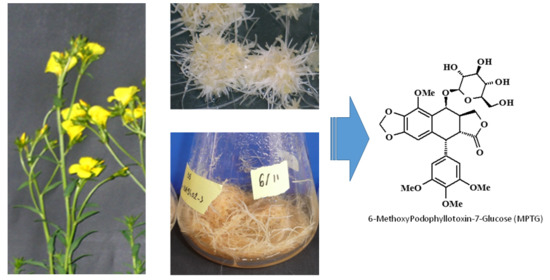
2.1. HR Induction and Molecular Characterization
2.2. Screening and Selection of Efficient HR Genotype
2.3. Improvement of Culture Conditions
2.4. Impact of Carbon Source and Concentrations
2.5. Impact of Phytohormones and Elicitors Treatments
2.6. Precursors Feeding
2.7. Permeation Experiments
3. Materials and Methods
3.1. Plant Materials
3.2. Agrobacterium Rhizogenes Strain and Culture Condition
3.3. Chemicals
3.4. Plant Transformation
3.5. DNA Extraction and PCR Analysis
3.6. RNA Extraction and Semi-Quantitative RT-PCR Analysis
3.7. HR Treatments
3.8. MPT and MPTG Purification
3.9. ATLs Extraction and Quantification by UPLC-HR-MS
3.10. Standard Curves for Quantification
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koulman, A.; Quax, W.J.; Pras, N. Podophyllotoxin and related lignans produced by plants. In Biotechnology of Medicinal Plants: Vitalizer and Therapeutic; Ramawat, K.G., Ed.; Science Publishers: New York, NY, USA, 2004; pp. 225–266. [Google Scholar]
- Ekstrom, K.; Hoffman, K.; Linne, T.; Eriksson, B.; Glimelius, B. Single-dose etoposide in advanced pancreatic and biliary cancer, a phase II study. Oncol. Rep. 1998, 5, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Sehested, M.; Jensen, P.B. Improved targeting of brain tumors using dexrazoxane rescue of topoisomerase II combined with supralethal doses of etoposide and teniposide. Clin. Cancer Res. 1998, 4, 1367–1373. [Google Scholar] [PubMed]
- Hammonds, T.R.; Denyer, S.P.; Jackson, D.E.; Irving, W.L. Studies to show that with podophyllotoxin the early replicative stages of herpes simplex virus type 1 depend upon functional cytoplasmic microtubules. J. Med. Microbiol. 1996, 45, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Wang, Y.-H.; Jin, Y.; Tian, X.; Zheng, Y.-T.; Luo, D.-Q.; Tu, Y.-Q. Synthesis and anti-HIV-1 activities of novel podophyllotoxin derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Bedir, E.; Khan, I.; Moraes, R.M. Bioprospecting for Podophyllotoxin. In Trends New Crop and New Uses; ASHS Press: Alexandria, VA, USA, 2002; pp. 545–549. [Google Scholar]
- Gordaliza, M.; García, P.A.; Miguel Del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, D.; Kumar, S. Biotechnological interventions for harnessing podophyllotoxin from plant and fungal species: Current status, challenges, and opportunities for its commercialization. Crit. Rev. Biotechnol. 2017, 37, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Alfermann, A.W. The production of cytotoxic lignans by plant cell cultures. Appl. Microbiol. Biotechnol. 2001, 55, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fuss, E. Lignans in plant cell and organ cultures: An overview. Phytochem. Rev. 2003, 2, 307–320. [Google Scholar] [CrossRef]
- Malik, S.; Bilba, O.; Gruz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Arroo, R.R.J.; Alfermann, A.W.; Medarde, M.; Petersen, M.; Pras, N.; Woolley, J.G. Plant Cell Factories as a Source for Anti-Cancer Lignans. Phytochem. Rev. 2002, 1, 27–35. [Google Scholar] [CrossRef]
- Vasilev, N.; Ebel, R.; Edrada, R.A.; Fuss, E.; Alfermann, A.W.; Ionkova, I.; Petrova, A.; Repplinger, M.; Schmidt, T.J. Metabolic Profiling of Lignan Variability in Linum species of Section Syllinum native to Bulgaria. Planta Media 2008, 74, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Hemmati, S.; Klaes, M.; Konuklugil, B.; Mohagheghzadeh, A.; Ionkova, I.; Fuss, E.; Wilhelm Alfermann, A. Lignans in flowering aerial parts of Linum species—Chemodiversity in the light of systematics and phylogeny. Phytochemistry 2010, 71, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in seeds of Linum species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghzadeh, A.; Hemmati, S.; Alfermann, A.W. Quantification of aryltetralin lignans in Linum album organs and in vitro cultures. Iran. Assoc. Pharm. Sci. 2006, 2, 47–56. [Google Scholar]
- Mohagheghzadeh, A.; Gholami, A.; Hemmati, S.; Ardakani, M.R.S.; Schmidt, T.J.; Alfermann, A.W. Root cultures of Linum species section Syllinum as rich sources of 6-methoxypodophyllotoxin. Z. Naturforsch. C J. Biosci. 2007, 62, 43–49. [Google Scholar] [CrossRef]
- Berlin, J.; Wray, V.; Mollenschott, C.; Sasse, F. Formation of β-Peltatin-A Methyl Ether and Coniferin by Root Cultures of Linum Flavum. J. Nat. Prod. 1986, 49, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.; Bedorf, N.; Mollenschott, C.; Wray, V.; Sasse, F.; Höfle, G. On the Podophyllotoxins of Root Cultures of Linum flavum. Planta Media 1988, 54, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Belma, K. Arytetralin lignans from Linum catharticum L. Biochem. Syst. Ecol. 1998, 26, 795–796. [Google Scholar] [CrossRef]
- Konuklugil, B.; Schmidt, T.J.; Alfermann, A.W. Accumulation of lignans in suspension cultures of Linum mucronatum ssp. armenum (Bordz.) Davis. Z. Naturforsch. C J. Biosci. 2001, 56, 1164–1165. [Google Scholar] [CrossRef]
- Lücking, B. Charakterisierung von Suspensionskulturen von Linum nodiflorum L.; Universities of Marburg and Halle-Wittenberg: Halle, Germany, 2001. [Google Scholar]
- Wink, M.; Alfermann, A.W.; Franke, R.; Wetterauer, B.; Distl, M.; Windhövel, J.; Krohn, O.; Fuss, E.; Garden, H.; Mohagheghzadeh, A.; et al. Sustainable bioproduction of phytochemicals by plant in vitro cultures: Anticancer agents. Plant Genet. Resour. 2005, 3, 90–100. [Google Scholar] [CrossRef]
- Berim, A.; Spring, O.; Conrad, J.; Maitrejean, M.; Boland, W.; Petersen, M. Enhancement of lignan biosynthesis in suspension cultures of Linum nodiflorum by coronalon, indanoyl-isoleucine and methyl jasmonate. Planta 2005, 222, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Oostdam, A.; Mol, J.N.M.; van der Plas, L.H.W. Establishment of hairy root cultures of Linum flavum producing the lignan 5-methoxypodophyllotoxin. Plant Cell Rep. 1993, 12, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Jafari, M.; Nejhad, N.; Hossenian, F. Podophyllotoxin and 6-methoxy podophyllotoxin Production in Hairy Root Cultures of Linum mucronatum ssp. mucronatum. Pharmacogn. Mag. 2014, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Chashmi, N.A.; Sharifi, M.; Yousefzadi, M.; Behmanesh, M.; Rezadoost, H.; Cardillo, A.; Palazon, J. Analysis of 6-methoxy podophyllotoxin and podophyllotoxin in hairy root cultures of Linum album Kotschy ex Boiss. Med. Chem. Res. 2013, 22, 745–752. [Google Scholar] [CrossRef]
- Vasilev, N.; Ionkova, I. Lignan accumulation in cell cultures of Linum strictum ssp. strictum L. Acta Pharm. 2004, 54, 347–351. [Google Scholar] [PubMed]
- Ono, N.N.; Tian, L. The multiplicity of hairy root cultures: Prolific possibilities. Plant Sci. 2011, 180, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Giovannini, A.; Ruffoni, B.; Bertoli, A.; Pistelli, L. Hairy Root Cultures for Secondary Metabolites Production. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: Boston, MA, USA, 2010; pp. 167–184. ISBN 978-1-4419-7347-4. [Google Scholar]
- Huynh Cong, L.; Dauwe, R.; Lequart, M.; Vinchon, S.; Renouard, S.; Fliniaux, O.; Colas, C.; Corbin, C.; Doussot, J.; Hano, C.; et al. Kinetics of glucosylated and non-glucosylated aryltetralin lignans in Linum hairy root cultures. Phytochemistry 2015, 115, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Kwok, K.H.; Doran, P.M. Development of Linum flavum hairy root cultures for production of coniferin. Biotechnol. Lett. 2003, 25, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Balen, B.; Leljak-Levanic, D.; Mihaijević, S.; Jelenić, S.; Jelaska, S. Formation of embryogenic callus in hairy roots of pumpkin (Cucurbita pepo L.). Vitr. Cell. Dev. Biol. Plant 2004, 40, 182–187. [Google Scholar] [CrossRef]
- Wichers, H.J.; Harkes, M.P.; Arroo, R.R.J. Occurrence of 5-methoxypodophyllotoxin in plants, cell cultures and regenerated plants of Linum flavum. Plant Cell Tissue Organ Cult. 1990, 23, 93–100. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Lopez, T.; Corbin, C.; Falguieres, A.; Doussot, J.; Montguillon, J.; Hagège, D.; Lainé, É. Secondary metabolite accumulation, antibacterial and antioxidant properties of in vitro propagated Clidemia hirta L. extracts are influenced by the basal culture medium. Comptes Rendus Chim. 2016, 19, 1071–1076. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic Growth Factor Requirements of Tobacco Tissue Cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of Mountain Laurel, Kalmia latifolia, by shoot tip culture. Int. Plant Prop. Soc. 1981, 30, 421–427. [Google Scholar]
- Mairet, F.; Sierra, J.; Glorian, V.; Villon, P.; Shakourzadeh, K.; Boitel-Conti, M. A new approach to define optimized range of medium composition for enhancement of hairy root production in fed-batch process. Bioprocess Biosyst. Eng. 2009, 32, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Srivastava, A.K.; Bisaria, V.S. Improved podophyllotoxin production by transformed cultures of Linum album. Biotechnol. J. 2008, 3, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2011, 16, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Kadkade, P. Growth and podophyllotoxin production in callus tissues of Podophyllum peltatum. Plant Sci. Lett. 1981, 25, 107–115. [Google Scholar] [CrossRef]
- Schmitt, J.; Petersen, M. Influence of methyl jasmonate and coniferyl alcohol on pinoresinol and matairesinol accumulation in a Forsythia × intermedia suspension culture. Plant Cell Rep. 2002, 20, 885–889. [Google Scholar] [CrossRef]
- Van Uden, W.; Pras, N.; Homan, B.; Malingré, T.M. Improvement of the production of 5-methoxypodophyllotoxin using a new selected root culture of Linum flavum L. Plant Cell Tissue Organ Cult. 1991, 27, 115–121. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Development of suspension culture of Podophyllum hexandrum for production of podophyllotoxin. Biotechnol. Lett. 2001, 23, 2063–2066. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Production of podophyllotoxin by plant cell cultures of Podophyllum hexandrum in bioreactor. J. Biosci. Bioeng. 2002, 93, 215–220. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Bernards, M.A.; He, L.; Davin, L.B.; Wooten, J.B.; Lewis, N.G. Lignification in cell suspension cultures of Pinus taeda: In situ characterization of a gymnosperm lignin. J. Biol. Chem. 1993, 268, 21088–21096. [Google Scholar] [PubMed]
- Ionkova, I. Effect of methyl jasmonate on production of ariltetralin lignans in hairy root cultures of Linum tauricum. Pharmacognosy Res. 2009, 1, 102–105. [Google Scholar]
- Klessig, D.F.; Malamy, J. The salicylic acid signal in plants. In Signals and Signal Transduction Pathways in Plants; Palme, K., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 203–222. ISBN 978-94-011-0239-1. [Google Scholar]
- Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Biosynthesis of lignans in Forsythia intermedia. Phytochemistry 1990, 29, 1841–1846. [Google Scholar] [CrossRef]
- Edahiro, J.-I.; Nakamura, M.; Seki, M.; Furusaki, S. Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of l-Phenylalanine into the medium. J. Biosci. Bioeng. 2005, 99, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Pras, N.; Malingré, T.M. On the improvement of the podophyllotoxin production by phenylpropanoid precursor feeding to cell cultures of Podophyllum hexandrum Royle. Plant Cell Tissue Organ Cult. 1990, 23, 217–224. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Update on Root Exudation and Rhizosphere Biology Root Exudation and Rhizosphere Biology. Plant Physiol. 2014, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kastell, A.; Knorr, D.; Smetanska, I. Exudation: An expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep. 2012, 31, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Bos, J.A.; Boeke, G.M.; Woerdenbag, H.J.; Pras, N. The large-scale isolation of deoxypodophyllotoxin from rhizomes of Anthriscus sylvestris followed by its bioconversion into 5-methoxypodophyllotoxin β-d-glucoside by cell cultures of Linum flavum. J. Nat. Prod. 1997, 60, 401–403. [Google Scholar] [CrossRef]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinié, R.; Corbin, C.; Montguillon, J.; Banuls, L.M.; Renouard, S.; Lamblin, F.; Dupré, P.; et al. Investigation of the Lignan Content in Extracts from Linum, Callitris and Juniperus Species in Relation to Their In Vitro Antiproliferative Activities. Planta Media 2017, 83, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus (Madison) 1990, 12, 13–15. [Google Scholar]
- Boitel-Conti, M.; Gontier, E.; Laberche, J.-C.; Ducrocq, C.; Sangwan-Norreel, B.S. Inducer effect of Tween 20 permeabilization treatment used for release of stored tropane alkaloids in Datura innoxia Mill. hairy root cultures. Plant Cell Rep. 1996, 16, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Homan, B.; Woerdenbag, H.J.; Pras, N.; Malingré, T.M.; Wichers, H.J.; Harkes, M. Isolation, Purification, and Cytotoxicity of 5-Methoxypodophyllotoxin, a Lignan from a Root Culture of Linum flavum. J. Nat. Prod. 1992, 55, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J.; Versluis-De Haan, G.G.; Marsman, J.W.; Harkes, M.P. Podophyllotoxin related lignans in plants and cell cultures of Linum flavum. Phytochemistry 1991, 30, 3601–3604. [Google Scholar] [CrossRef]






| Cell Lines | MPT-G mg·g−1 DW | MPT mg·g−1 DW | PPT-G mg·g−1 DW | PPT mg·g−1 DW | DPT mg·g−1 DW | Growth Index Days 0–20 |
|---|---|---|---|---|---|---|
| in vitro WT Lf root | 4.9 ± 0.5 | 1.7 ± 0.1 | 1.0 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.1 | 1.3 ± 0.1 |
| HRLF15.1 | 21.9 ± 4.4 | 8.3 ± 1.6 | 2.3 ± 0.4 | 0.6 ± 0.1 | 1.3 ± 0.4 | 3.6 ± 0.1 |
| HRLF15.2 | 22.6 ± 5.7 | 7.5 ± 1.0 | 2.9 ± 0.8 | 0.8 ± 0.3 | 1.0 ± 0.2 | 4.0 ± 0.2 |
| HRLF15.3 | 5.8 ± 0.1 | 5.1 ± 0.6 | 1.6 ± 0.8 | 0.3 ± 0.1 | 0.6 ± 0.1 | 3.5 ± 0.1 |
| HRLF15.4 | 6.1 ± 0.2 | 3.0 ± 0.3 | 1.3 ± 0.3 | 0.2 ± 0.1 | 0.9 ± 0.1 | 3.4 ± 0.1 |
| HRLF15.5 | 5.8 ± 1.3 | 1.5 ± 0.1 | 2.7 ± 0.5 | 0.4 ± 0.2 | 0.5 ± 0.3 | 3.8 ± 0.1 |
| HRLF94.1 | 9.7 ± 0.6 | 3.5 ± 0.2 | 1.2 ± 0.3 | 0.2 ± 0.1 | 1.0 ± 0.4 | 5.0 ± 0.2 |
| HRLF94.2 | 24.1 ± 3.1 | 8.4 ± 0.4 | 4.9 ± 0.3 | 0.6 ± 0.2 | 1.6 ± 0.4 | 5.9 ± 0.2 |
| HRLF94.3 | 5.9 ± 0.2 | 5.6 ± 1.2 | 2.0 ± 0.6 | 0.3 ± 0.1 | 0.8 ± 0.2 | 5.2 ± 0.3 |
| HRLF94.4 | 4.9 ± 1.4 | 6..3 ± 0.7 | 1.5 ± 0.4 | 0.2 ± 0.1 | 0.7 ± 0.2 | 5.0 ± 0.2 |
| HRLF94.7 | 20.4 ± 1.3 | 3.2 ± 1.2 | 2.0 ± 0.5 | 0.3 ± 0.1 | 0.8 ± 0.2 | 4.4 ± 0.2 |
| HRLF94.8 | 4.1 ± 0.3 | 6.9 ± 1.1 | 1.2 ± 0.3 | 0.2 ± 0.1 | 0.7 ± 0.2 | 4.9 ± 0.2 |
| HRLF94.9 | 12.4 ± 1.9 | 6.3 ± 2.0 | 1.8 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.2 | 4.8 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans. Int. J. Mol. Sci. 2018, 19, 990. https://doi.org/10.3390/ijms19040990
Renouard S, Corbin C, Drouet S, Medvedec B, Doussot J, Colas C, Maunit B, Bhambra AS, Gontier E, Jullian N, et al. Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans. International Journal of Molecular Sciences. 2018; 19(4):990. https://doi.org/10.3390/ijms19040990
Chicago/Turabian StyleRenouard, Sullivan, Cyrielle Corbin, Samantha Drouet, Barbara Medvedec, Joël Doussot, Cyril Colas, Benoit Maunit, Avninder S. Bhambra, Eric Gontier, Nathalie Jullian, and et al. 2018. "Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans" International Journal of Molecular Sciences 19, no. 4: 990. https://doi.org/10.3390/ijms19040990